1.1 NVP and its polymers
¡¡¡¡PVP is a non-ionic water-soluble polymer fine chemical with excellent performance and wide applications. It is polymerized from NVP under certain conditions and is the most distinctive and extensively studied fine chemical among N-vinylamide functional group polymers. Since Reppe, a German acetylene chemist, first disclosed the patent for synthesizing NVP and its polymer PVP using acetylene as raw material in 1938, there has been more than 60 years of research, production, and application history. It has now developed into three major categories: non-ionic, cationic, and anionic, industrial grade, pharmaceutical grade, and food grade, with a total of more than ten varieties and molecular weights ranging from thousands to over one million. With its excellent and unique properties, it is widely used in industrial and agricultural production, people's lives, and related scientific research departments. It still showcases its thriving status and continued development prospects with hundreds of publications every year.
1.1.1 Properties of NVP
¡¡¡¡1. Physical properties
NVP is a colorless or pale yellow, slightly odorous transparent liquid at room temperature, easily soluble in water, and its main physical properties are as follows:
¡¡¡¡Density£º 1.04£¨25¡æ£©
¡¡¡¡Melting point£º 13.5¡æ
¡¡¡¡Boiling point£º 148¡æ£¨13332.24Pa£©£¬58¡«65¡æ£¨13.3¡«26.64Pa£©
¡¡¡¡Flash point£º 98.33¡æ
¡¡¡¡Refractive index£º
NVP has excellent solution properties. In addition to being soluble in water, it is also soluble in many organic solvents such as methanol, ethanol, propanol, isopropanol, chloroform, glycerol, tetrahydrofuran, vinyl acetate, etc. It can also be dissolved in aromatic solvents such as toluene.
¡¡¡¡Generally speaking, NVP has good solubility in strongly polar solvents, but poor solubility in non-polar solvents, as shown in Table 1.1.
Table 1.1 Solubility of NVP in various solvents
Solvent | Water | Ethanol | Benzene | Toluene | Acetone | Chloroform | 1,4-dioxane |
Solubility | ¡Ì | ¡Ì | ¡Ì | ¡Á | ¡Á | ¡Ì | ¡Á |
Solvent | DMF | THF | Xylene | Pentanol | Cyclohexane | Ethyl acetate | Butyl Acrylate |
Solubility | ¡Ì | ¡Á | ¡Á | ¡Á | ¡Á | ¡Á | ¡Á |
Solvent | Methanol | Propanol | CYCLOHEXANONE | Pentane | Formic acid | Dichloromethane | Methylcyclohexane |
Solubility | ¡Ì | ¡Ì | ¡Á | ¡Á | ¡Ì | ¡Ì | ¡Á |
Solvent | Acet- | Propionic acid | Petroleum ether | Turpentine | Chlorobenzene | Carbon tetrachloride | Ethyl vinyl ether |
Solubility | ¡Ì | ¡Ì | ¡Á | ¡Á | ¡Á | ¡Á | ¡Á |
Note: "¡Ì" indicates solubility; "X" indicates poor solubility.
2. Chemical properties
¡¡¡¡The molecular formula of NVP is C6H9NO, and its structure is£º
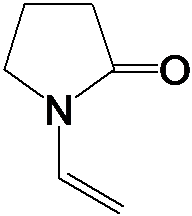
¡¡¡¡The molecule of NVP is a five membered ring containing an N atom, belonging to the class of lactam compounds, with an ethylene group attached to the N atom. The unique molecular structure of NVP endows it with some special chemical properties, among which the most important are its easy polymerization and easy hydrolysis.
¡¡¡¡NVP can undergo polymerization reaction to obtain PVP under appropriate initiators or light exposure, and the reaction equation is as follows£º
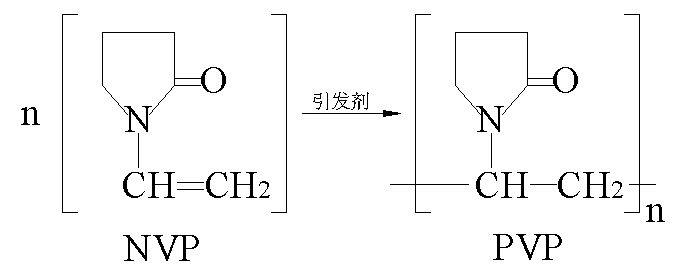
¡¡¡¡Even in the absence of an initiator, NVP may undergo varying degrees of self polymerization during prolonged storage or transportation due to vibration, which can affect its quality. Therefore, commercially available NVP products generally contain polymerization inhibitors, which can be removed by vacuum distillation or activated carbon adsorption during use.
¡¡¡¡Another important chemical property of NVP is that it is easily hydrolyzed in the presence of acidic or alkali metal ions, producing pyrrolidone and acetaldehyde. The hydrolysis process is shown in Figure 1.1 [2].
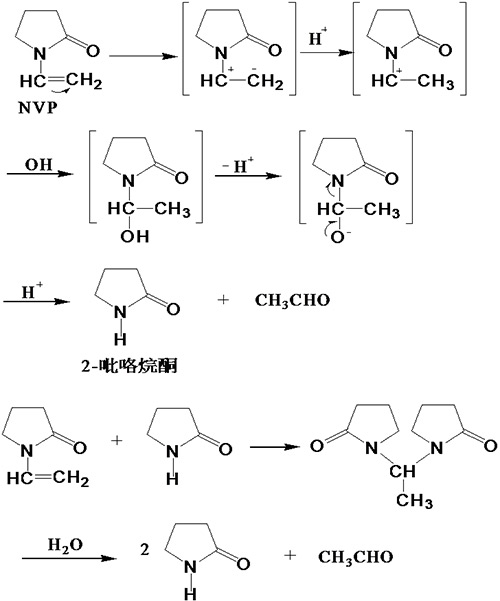
Figure 1.1 Hydrolysis of NVP
¡¡¡¡Due to the easy hydrolysis of NVP, two points should be noted in the production and use of NVP: firstly, when synthesizing NVP, it is necessary to completely remove water to ensure that the product does not contain any moisture; Secondly, during storage and transportation, the product should be made neutral or weakly alkaline to prevent hydrolysis and self polymerization reactions. The usual method is to add 0.1% alkali (such as sodium hydroxide, ammonia, or low molecular weight amines).
1.1.2 Synthesis of NVP
¡¡¡¡The polymer monomer NVP of PVP was synthesized by chemist W. from BASF in Germany in 1938 Reppe was synthesized for the first time [1]. He uses acetylene as the starting material, undergoes a series of chemical reactions, and finally obtains NVP. This synthesis method is called acetylene method, also known as Reppe method.
¡¡¡¡This method uses acetylene and formaldehyde as starting materials, and undergoes five steps of reaction including aldehyde addition of acetylene, catalytic hydrogenation, catalytic dehydrogenation to form rings, ammonolysis, and acetylene addition, to finally obtain NVP. The principle can be represented by the reaction equation shown in Figure 1.2 [1,3-6]£º
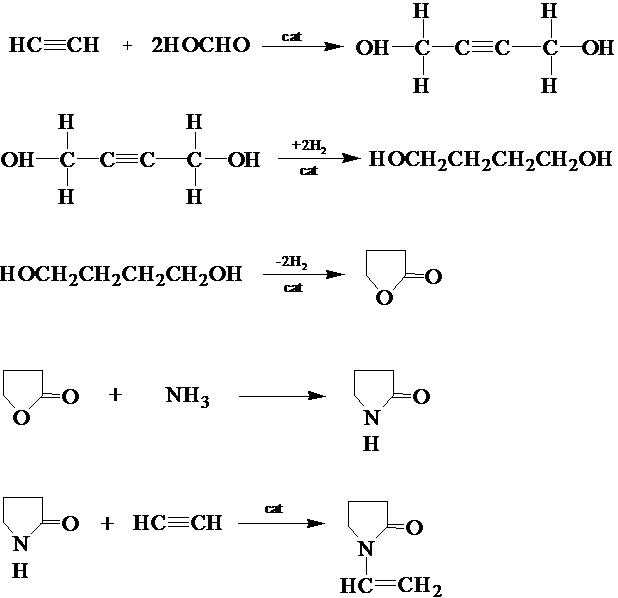
Figure 1.2 Acetylene synthesis route of NVP
¡¡¡¡The acetylene method for producing NVP has become quite mature and is one of the main methods for industrial production of NVP in the world today. The main advantages of this process are mature technology, cheap and easy to obtain raw materials, etc. The main disadvantages are long process flow, large fixed capital investment, strict operating conditions, and the risk of explosion in the main raw material acetylene.
¡¡¡¡In recent decades, technology workers have been improving the acetylene process. We have also researched and developed or are currently working on developing other NVP synthesis and production methods. After decades of development, various methods for synthesizing NVP have emerged, such as gamma butyrolactone method, pyrrolidone method, maleic anhydride method, succinic acid method, etc. [7-13]. But these methods are still under further research and require further breakthroughs.
1.1.3 Aggregation reaction of NVP
¡¡¡¡NVP monomers have no practical application value in industry. Only by polymerizing or copolymerizing NVP into polymer compounds with a certain structure, composition, and molecular weight can they be applied in industry.
NVP can be homopolymerized or copolymerized with other monomers; The obtained polymer can be linear or cross-linked.
1. Homopolymer
¡¡¡¡NVP monomers are highly prone to polymerization reactions. Heating the NVP monomer to above 140 ¡æ or adding initiators to the NVP monomer can easily induce homopolymerization of NVP, resulting in the formation of polyvinylpyrrolidone (PVP). Initiators include cationic initiators such as BF3 [14-15]; Anionic initiators, such as potassium salts of amides; Free radical initiators, such as peroxides, azo compounds, etc. Similar to the synthesis methods of other polymers, the polymerization methods of NVP include bulk polymerization, solution polymerization, and suspension polymerization.
¡¡¡¡Due to the gradual increase in viscosity of the reaction system during the polymerization process, difficulty in polymer diffusion, difficulty in removing reaction heat, and local overheating, the quality of the obtained product is poor and cannot meet the requirements of commercial products. Therefore, it has no practical application value in industrial production. Suspension polymerization is also rarely reported. At present, the polymerization of NVP in industry is generally carried out by solution polymerization. The solvents used for solution polymerization include water, ethanol, isopropanol, benzene, methanol, ethyl acetate, etc. The most commonly used is water.
¡¡¡¡The polymerization mechanism of NVP in water and organic solvents is different. The polymerization mechanism in aqueous solution is shown in Figure 1.3 (using hydrogen peroxide as the initiator)[16,17]£º
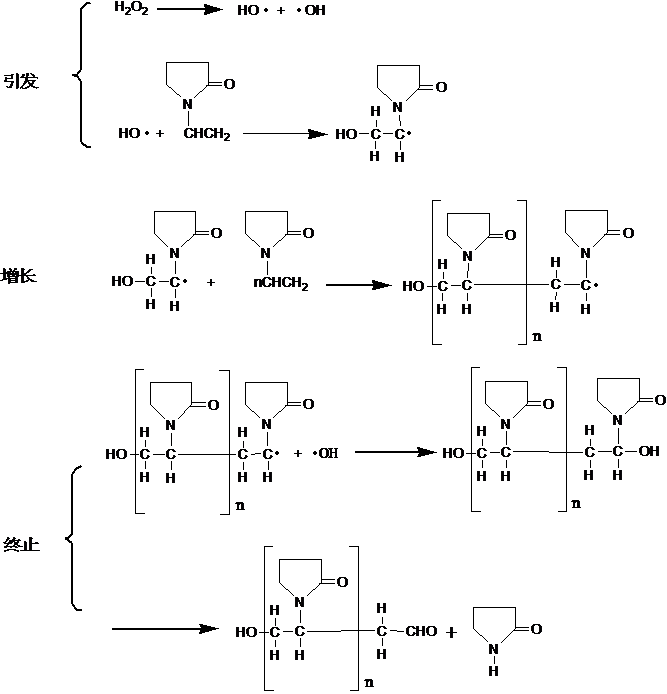
Figure 1.3 Aggregation mechanism of NVP in water
¡¡¡¡When NVP is polymerized in aqueous solution, long-chain free radicals are usually terminated by binding with initiator free radicals, and during the chain termination stage, the cleavage reaction of the pyrrolidone ring occurs simultaneously. Therefore, aldehyde groups are often found to be one of the end groups of PVP. The cracking reaction also generates a small amount of pyrrolidone, so the polymer obtained by aqueous solution polymerization is not particularly pure.
¡¡¡¡Due to the fact that long-chain free radicals terminate through binding with initiator free radicals in aqueous solution polymerization, the amount of initiator has a particularly significant impact on the molecular weight of the polymer [18].
¡¡¡¡The dynamic chain length is the ratio of the growth reaction rate to the termination reaction rate£º
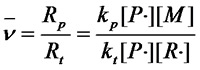 £¨Equation 1.1£©
£¨Equation 1.1£©

else
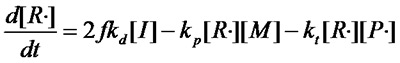 £¨Equation 1.2£©
£¨Equation 1.2£©
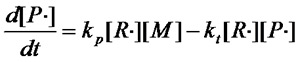 £¨Equation 1.3£©
£¨Equation 1.3£©
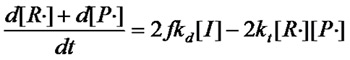 £¨Equation 1.4£©
£¨Equation 1.4£©
¡¡¡¡According to the steady-state assumption, the concentration of free radicals in the polymerization system remains constant, so
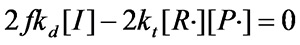 £¨Equation 1.5£©
£¨Equation 1.5£©
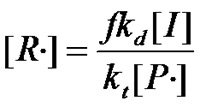 £¨Equation 1.6£©
£¨Equation 1.6£©
¡¡¡¡Substituting equation 1.6 into equation 1.1 yields£º
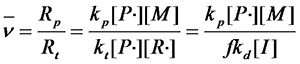 £¨Equation 1.7£©
£¨Equation 1.7£©
¡¡¡¡Equation 1.7 and the kinetic chain length of the typical double base termination method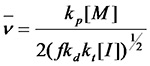 Compared to that, the effect of initiator concentration on molecular weight is much greater.
Compared to that, the effect of initiator concentration on molecular weight is much greater.
¡¡¡¡Moreover, NVP has hydrogen bonding with water, which also affects its reactivity. Figure 1.4 shows the relationship between initial reaction rate and NVP concentration in water[19]¡£
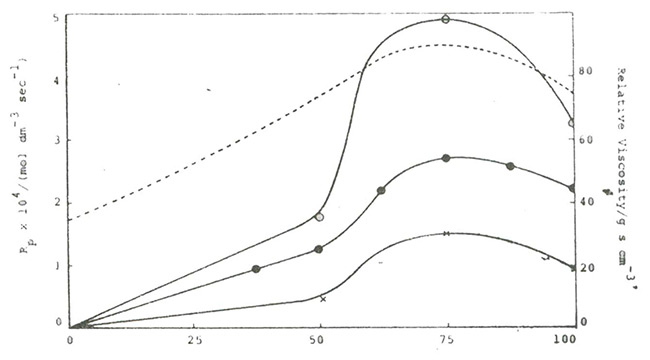
Figure 1.4 Effect of Hydrogen Bonds on the Reaction Rate of NVP Polymerization
¡¡¡¡From the graph, it can be seen that when the NVP concentration is 75% (vol), the corresponding molar ratio of NVP to water is 1:2, and the polymerization reaction rate is the highest, and the system viscosity is also the highest at this time. This hydrogen bonding can enhance the reactivity of NVP, thereby increasing the reaction rate. However, excessive water dilutes the hydrogen bond between NVP and water, resulting in a decrease in reaction rate when NVP concentration is low.
¡¡¡¡When NVP undergoes polymerization reaction in organic solvents such as alcohols, the reaction mechanism is relatively complex, as shown in Figure 1.5.
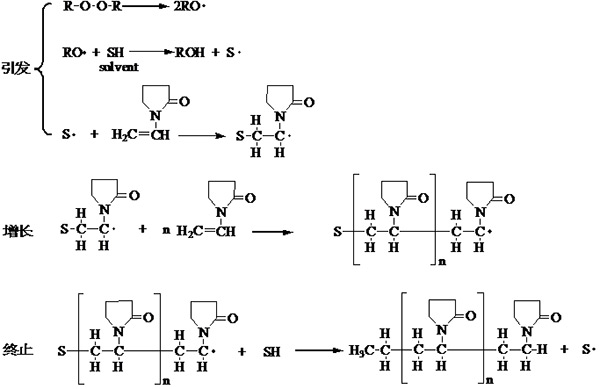
Figure 1.5 Aggregation mechanism of NVP in organic solvents
¡¡¡¡When conducting polymerization in organic solvents, the initiator free radicals react with the solvent to generate solvent free radicals that initiate monomer polymerization. The termination of the chain is achieved by transferring to the solvent chain, obtaining a hydrogen atom end group and another solvent free radical, which continue to initiate the polymerization reaction. Due to the absence of cleavage of the pyrrolidone ring during the termination reaction, as in polymerization in aqueous solution, the prepared polymer is relatively pure; There is no aldehyde end group present, and it is more stable than aqueous solution polymerization under oxidative conditions. However, due to chain transfer reactions, polymerization in organic solvents generally only yields lower molecular weight polymers. To synthesize high molecular weight polymers, small doses of initiators must be used in aqueous solutions.
2. Co polymerization
¡¡¡¡In addition to homopolymerization, NVP can also undergo copolymerization reactions with other monomers containing vinyl structures to generate copolymers with various properties.
The monomers that can undergo copolymerization reaction with NVP are listed in Table 1.2.
Table 1.2 Monomers that can undergo copolymerization reaction with NVP | |
Esters | Dimethylaminoethyl methacrylate acrylic ester, acrylic acetate, isobutylene ester, diacetate acrylic ester, methyl methacrylate, vinyl acetate, ethylene dicarbonate, vinyl propionate, isopropylacrylate, triallyl isocyanurate, dimethylaminoethyl isobutyl acrylate, dimethylaminoethyl methyl methacrylate, vinyl acrylate, tributyl acrylate, isooctyl acrylate, etc |
Ether class | Vinyl phenyl ether, vinyl isopropyl ether, vinyl cyclohexyl ether, vinyl butyl ether, etc |
Hydrocarbons | Ethylene, styrene, hexadecene |
Amide class | N-vinylbenzamide, acrylamide, vinyl caprolactam |
Halogenated hydrocarbons | Chloroethylene, trichloroethylene |
Alcohols | Allyl alcohol |
Bifunctional group (cross-linked polymerization) | Methylene bisacrylamide, divinylbenzene |
Other categories | Acrylonitrile, maleic anhydride, vinyltrimethylsilane, vinyltrimethoxysilane, vinylimidazole, etc |
¡¡¡¡Although free radical polymerization can produce many copolymers of NVP, currently only a few are widely produced in industry, such as NVP/vinyl acetate, NVP/vinyl propionate, mainly used in cosmetics, printing and dyeing auxiliaries, and food additives.
Table 1.3 Co polymerization parameters of some unsaturated monomers for NVP (M1)[20] | ||||
Monomer£¨M2£© | r1 | r 2 | Q | e |
Ethylene dicarbonate | 0.4 | 0.7 | ¡ª | ¡ª |
Vinyl Laurate | 1.15¡«1.3 | 0.01 | ¡ª | ¡ª |
Vinyl Acetate | 2.0 | 0.24 | ¡ª | ¡ª |
Methyl methacrylate | 0.005 | 4.7 | 0.074 | -1.33 |
Acrylic diacetate | 0.92 | 0.94 | 0.096 | -1.27 |
Acrylic acetate | 1.6 | 0.17 | ¡ª | ¡ª |
N-vinylbenzeneimide | 0.35 | 0.04 | 0.088 | 0.37 |
Maleic anhydride | 0.16 | 0.08 | ¡ª | ¡ª |
Allyl alcohol | 1.0 | 0 | ¡ª | ¡ª |
Acrylonitrile | 0.06 | 0.18 | ¡ª | ¡ª |
Syrene | 0.045 | 15.7 | 0.087 | -1.22 |
Trichloroethene | 0.54 | <0.01 | ¡ª | ¡ª |
Vinyl chloride | 0.38 | 0.53 | 0.035 | -1.07 |
Vinyl phenyl ether | 4.43 | 0.22 | ¡ª | ¡ª |
Vinyl isopropyl ether | 1.68 | ¡ª | ¡ª | ¡ª |
Vinyl cyclohexyl ether | 3.84 | ¡ª | ¡ª | ¡ª |
Vinyl butyl ether | 3.30 | 0.205 | 0.067 | -1.12 |
3. Cross linked polymerization
¡¡¡¡The linear polymer PVP of NVP is a water-soluble polymer, but its cross-linked polymer cannot dissolve in water and organic solvents.
¡¡¡¡There are three methods to synthesize cross-linked PVP:
¡¡¡¡a) PVPP with low crosslinking degree can be obtained by treating PVP with persulfate, hydrazine or hydrogen peroxide, or treating PVP with ¦Á, ¦Ø - diene in the presence of peroxide, and the product is soft gel [21-23].
¡¡¡¡b) Free radical polymerization with added crosslinking agent [24]. Common crosslinking agents include divinylbenzene, N, N '- methylenebisacrylamide, vinyl dimethacrylate, ¦Á, ¦Ø - dienes, etc. When synthesizing PVPP using this method, the crosslinking degree of PVPP can be controlled by controlling the amount of crosslinking agent. However, this method can only obtain PVPP with moderate crosslinking degree. To obtain PVPP with high crosslinking degree, a large amount of crosslinking agent needs to be added, which is not cost-effective in industry.
c) Popcorn aggregation [25-28]. The highly cross-linked PVPP is synthesized using this method.
1.1.4 Properties of PVP [29]
¡¡¡¡PVP is a water-soluble polymer compound that possesses general properties of water-soluble polymer compounds, such as colloid protection, film-forming ability, adhesion, hygroscopicity, solubilization or coagulation, and chelating ability with certain compounds. But the most distinctive feature of PVP is its excellent solubility and physiological inertness. PVP is soluble in both water and most organic solvents, non-toxic and harmless, with good physiological compatibility.
¡¡¡¡1. Determination and characterization of molecular weight
¡¡¡¡PVP products are divided into several grades based on their molecular weight, generally represented by the K value of the Fikentscher method. The most commonly used method for determining the K value is to use a capillary viscometer to measure the relative viscosity ¦Ç of PVP aqueous solution
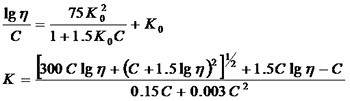
In the formula: K0 Fikentscher constant K=1000 K0;
C The number of grams of PVP dissolved in 100ml;£»
¦Ç relative viscosity
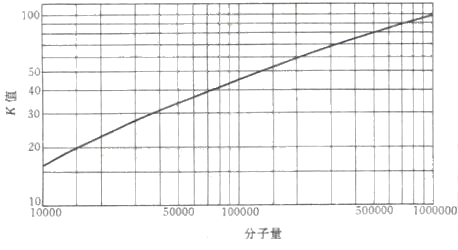
Figure 1.6 Correspondence between K value and molecular weight of PVP
2. Physical properties
¡¡¡¡The melting heat of PVP in water is -4.8 kJ/mol, with a refractive index. The solid density of PVP is 1.25 ¡Á 103kg/m3, but due to its loose structure, its bulk density varies from 0.1 to 0.6g/ml depending on the molecular weight and drying method.
¡¡The glass transition temperature of PVP also increases with the increase of molecular weight, and its limit value is about 180 ¡æ, as shown in Figure 1.7: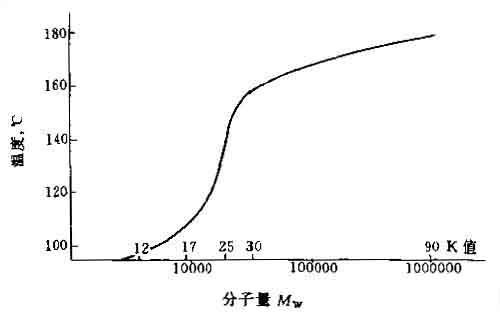
Figure 1.7 Glass transition temperature of PVP
¡¡¡¡The X-ray scattering angles 2 ¦È of PVP are 10 ¡ã~26 ¡ã and 10 ¡ã~40 ¡ã, respectively, indicating that PVP has an amorphous structure.
3. Solubility and solution characteristics
¡¡¡¡Due to the presence of both hydrophilic and oleophilic groups in PVP molecules, they can interact with many solvents, making them soluble in both water and organic solvents such as alcohols, carboxylic acids, amines, and halogenated hydrocarbons. The solubility of PVP in water is limited only by its own viscosity.
Examples of organic solvents that can dissolve more than 10% PVP at room temperature are shown in Table 1.4, and examples of organic solvents that can dissolve less than 1% PVP at room temperature are shown in Table 1.5.
¡¡¡¡From Tables 1.4 and 1.5, it can be seen that PVP is generally soluble in solvents with strong polarity, but not in solvents with weaker polarity. However, when a co solvent is present, PVP is soluble in non-polar solvents such as hydrocarbons.
Table 1.4 Organic solvents that easily dissolve PVP | |
| Alcohols | Methanol, Ethanol, Propanol, Isopropanol, Butanol, Isobutanol, Pentanol, Cyclohexanol, Methylcyclohexanol, Phenol, Ethylene glycol, Propylene glycol, Butanediol, Glycerol, Diacetone alcohol |
Acids | Formic acid, Acetic acid, Propionic acid |
lactones | ¦Ã£Butyrolactone |
Ether alcohol | Ethylene glycol ether, Diethylene glycol, Triethylene glycol, 1,6-Hexanediol, Polyethylene glycol 400, 2,2 'Thiodiethanol |
Esters | Ethyl lactate |
Ketones | Methyl cyclohexanone, Cyclohexanone (hot) |
lactams | 2-Pyrrolidone, N-Methylpyrrolidone, N-Vinylpyrrolidone |
Amine | Butylamine, Cyclohexylamine, Aniline, Ethylenediamine, Pyridine, Morpholine, Ethanolamine, Diethanolamine, Triethanolamine, Aminoethyl ethanolamine, 2-Hydroxyethyl morpholine, 2-Amino-2-Methylpropanol |
Nitrohydrocarbons | Nitromethane, Nitroethane |
Table 1.5 Organic solvents that are not easily soluble in PVP | |
Hydrocarbons | Benzene, Toluene, Xylene, Petroleum ether, Tetrahydronaphthalene, Pentane, Hexane, Heptane, Dry cleaning solvent oil, Kerosene, Solvent oil, Mineral oil, Cyclohexane, Methylcyclohexane, Turpentine oil |
Ether class | Dioxane, Diethyl ether, Dimethyl ether, Ethyl vinyl ether, Isobutyl vinyl ether, Tetrahydrofuran |
Chlorine containing compounds | Carbon tetrachloride, Chlorobenzene |
Ketones | Acetone, 2-Butanone, Cyclohexanone |
Esters | Ethyl acetate, Isobutyl acetate |
¡¡¡¡In a larger range, the viscosity of PVP aqueous solution is independent of pH value, and only undergoes significant changes in extreme cases: concentrated hydrochloric acid increases the viscosity of the solution, and concentrated alkali causes PVP to precipitate.
¡¡¡¡The viscosity of PVP varies greatly in other organic solvents, as shown in Table 1.6. For some organic solvents, the reason why the solution viscosity increases is that PVP causes gel after mixing with PVP.
Table 1.6 Viscosity of 10% PVP K30 solution in different solvents at 25 ¡æ | |||||||
Solvent | Dichloromethane | N-Methylpyrrolidone | Water | Ether | Propyl glycol | Glycol | Isopropanol |
Viscosity£¬mm2/s | 3 | 8 | 5 | 12 | 261 | 95 | 12 |
Solvent | Diethylene glycol | Triethanolammonium | Ethanol | Acetic acid | Cyclohexanol | Glycerol | Butanediol |
Viscosity£¬mm2/s | 165 | 666 | 5 | 12 | 376 | 2046 | 425 |
¡¡¡¡Dissolving PVP in NaCl aqueous solution (0.2mol or 2mol), its behavioral characteristics such as viscosity are only slightly different from PVP aqueous solution, which is the excellent characteristic that distinguishes PVP from most other polymers.
¡¡¡¡PVP also has a unique solubility in mixed solvents. The phase diagram of the ternary system of PVP/acetone/water is shown in Figure 1.9. From the phase diagram, it can be seen that acetone is almost insoluble in PVP. Therefore, when a large amount of acetone is added to the aqueous solution of PVP, some PVP will precipitate with acetone, forming two phases. The position of the three-phase eutectic point tends towards an increase in the proportion of acetone as the K value decreases.
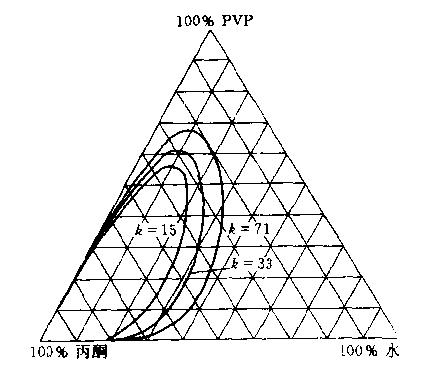
Figure 1.9 Phase diagram of PVP/acetone/water ternary system (25 ¡æ)
4. Surface activity
¡¡¡¡From a structural perspective, the long molecular chain of PVP contains both hydrophilic and polar groups of lactam groups, as well as lipophilic non-polar carbon chains. This molecular structure of PVP gives it surface activity. Although its ability to reduce surface or interfacial tension is smaller than that of low molecular weight surfactants and its permeability is weaker, its adsorption on solid surfaces and the three-dimensional shielding ability formed by hydrophilicity make solid particles have excellent dispersion stability. In addition, its ability to form hydrogen bonds with many organic-inorganic compounds gives it coagulation or solubilization properties. The former enables it to clarify and stabilize in alcoholic beverages containing polyphenols, while the latter is widely used in co precipitation to prepare solid drug dispersions and improve the bioavailability of insoluble drugs. The comprehensive characteristics of these surface active substances make PVP one of the main varieties of polymer surfactants.
5. Moisture absorption
¡¡¡¡PVP has strong hygroscopicity, and its saturated moisture content at different relative humidities is shown in Figure 1.10.
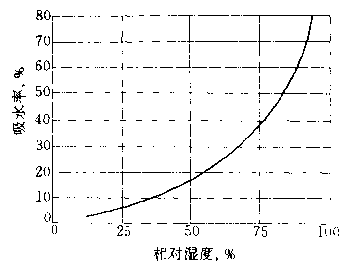
Figure 1.10 Saturated Moisture Content of PVP
¡¡¡¡Thermochemical studies have shown that each NVP unit binds approximately 0.5 molecules of water, which is similar to the water absorption of proteins.
6. Complexation
¡¡¡¡PVP is a polymer with high "solubility" ability, which is due to the high polarity and amide groups that can accept hydrogen bonds in its molecular structure, as well as non-polar groups. This ability enables PVP to form complexes with many substances, especially compounds containing hydroxyl, carboxyl, amino, and other active hydrogen atoms.
7. Chemical stability
¡¡¡¡Under normal circumstances, solid PVP is very stable and remains unchanged after being heated in air at 100 ¡æ for 16 hours. The aqueous solution of PVP is also very stable under normal circumstances. When free of other components, there is no sign of precipitation in PVP aqueous solution within the range of 0-100 ¡æ. However, if heated for too long, stored for too long, or the pH value is in the acidic range, the PVP solution will turn slightly pale yellow. Adding various salts containing multivalent anions, such as sodium metasilicate and sodium tripolyphosphate, to PVP aqueous solution will cause precipitation. However, high molecular weight PVP, whether in aqueous solution or dry powder state, will degrade if stored for too long.
8. Biological characteristics
¡¡¡¡PVP is non-toxic, has excellent physiological inertia, does not participate in human metabolism, and has excellent biocompatibility, without causing any irritation to the skin, mucous membranes, eyes, etc.
1.1.5 Purpose of PVP[30]
¡¡¡¡1. Medicine
¡¡¡¡Shortly after its emergence, PVP was used as a plasma expander and saved numerous wounded soldiers during World War II.
¡¡¡¡Nowadays, PVP, cellulose derivatives, and acrylic compounds have become the three main synthetic drug excipients and are widely used worldwide. It can be used as a binder for tablets and granules, a co solvent and stabilizer for injections, a dispersant for liquid formulations, a coating film-forming agent and pigment dispersant, a co precipitant for insoluble drugs, a delay agent for eye drops, lubricant, etc. Cross linked insoluble PVP can also be used as a drug disintegrant for tablets.
PVP has many applications as a non excipient, among which the most common is PVP-I used as a disinfectant for sterilization.
¡¡¡¡2. Cosmetics
¡¡¡¡PVP has many functions in cosmetics, mainly including stabilizing and tackifying lotion, suspension and other dispersion systems, film forming in hair care and styling products, gel in gel products, lubrication and moisturizing in skin care products, foam stabilizing in foam products, and pigment stabilizing agent, deodorant, fragrance preserving agent, and mild agent in various cosmetics formulations.
¡¡¡¡3. Brewing and beverage industry
¡¡¡¡PVP and insoluble PVP can be used as clarifying agents and stabilizers for beer, fruit wine, and fruit juice in the brewing and beverage industries. PVP can complex with polyphenolic substances in these alcoholic beverages, thereby removing substances that can make them cloudy.
¡¡¡¡4. Paint and pigment industry
¡¡¡¡PVP is widely used in industrial processes or products such as coatings, pigments, inks, polymer synthesis, and processing due to its excellent solubility, film-forming properties, physiological inertness, surface activity, and chelating ability.
¡¡¡¡5. Adhesive
¡¡¡¡PVP has special adhesion to glass, metal, and plastic surfaces; Combined with its hydrophilicity, dispersion stability, non thixotropy, and thickening properties, it is widely used in various adhesive formulations, such as solid glue sticks, pressure-sensitive adhesives, and re wetting adhesives.
¡¡¡¡6. Other
¡¡¡¡In addition to the aforementioned uses of PVP, PVP is also applied in other fields such as oil extraction and papermaking. Due to its excellent performance, PVP has been continuously discovered to have many new applications in recent years; By adopting new polymerization techniques and copolymerization methods, the properties of polymers can be further improved, thus opening up some new application areas.